Astrazeneca Covid 19 Vaccine Results
AZD1222 COVID-19 Vaccine - Executive Summary AstraZeneca committed to a partnership with Oxford University to ensure broad and equitable vaccine access globally not for profit during the pandemic. A UK study found that mixing different brands of COVID-19 vaccines specifically mRNA vaccines from AstraZeneca and Pfizer could give better protection that could last for several years.
Explained Here Are The Key Takeaways From Oxford S Covid 19 Vaccine Error Explained News The Indian Express
Read the 16 April 2021 statement of the WHO Global Advisory Committee on Vaccine Safety on AstraZeneca COVID-19 vaccine which covers reports of very rare side.
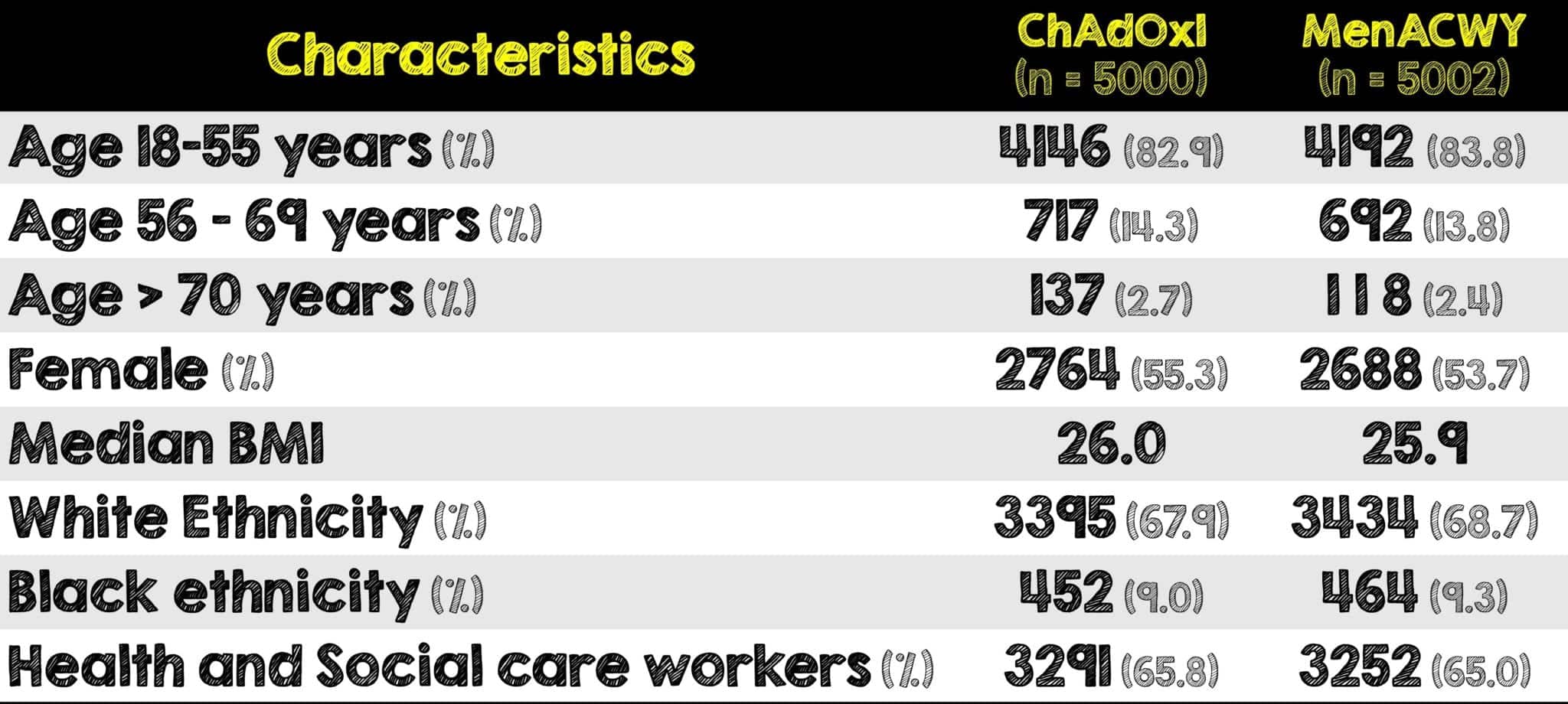
Astrazeneca covid 19 vaccine results. As of 19 April 2021 the AstraZeneca vaccine is safe and effective at protecting people from the extremely serious risks of COVID-19 including death hospitalization and severe disease. Clinical trial data show Modernas vaccine is. Clinical trials for COVID-19 Vaccine AstraZeneca did not include people with immunocompromise but many people with such conditions have now been vaccinated worldwide.
AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the. SeventyFour iStock. Modernas COVID-19 vaccine is built on a similar biotech platform messenger RNA to the Pfizer-BioNTech shots.
Study In a real-world study in the UK two doses of COVID-19 vaccines. The AstraZeneca COVID-19 vaccine showed an effectiveness of about 62 in preventing symptomatic COVID-19 disease beginning 2 weeks after the second dose. Both seem less effective than the two-shot vaccines made by PfizerBioNTech and Moderna which exceeded 90 efficacy.
Most COVID-19 vaccines require two doses and the usual. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled. Pharma Two shots of Pfizer AstraZeneca COVID-19 vaccines effective against Delta variant.
After discovering that especially younger women who. AstraZeneca COVID-19 vaccine doses have better efficacy when given 12 weeks apart study finds. Results suggested a 45-week delay between the first and second dose lent up to an 18-fold increase in antibody response per data taken 28 days post-second dose.
Vaccine immunogenicity efficacy and safety were demonstrated in four Phase I-III non-IND trials in UK Brazil South Africa. Is it safe to have more than one type of COVID-19 vaccineA trial has now addressed that question as well as what effect combining different vaccine types has on immunity. The AstraZeneca COVID-19 vaccine is not yet approved in the United States because the FDA has asked AstraZeneca to show results from a large-scale trial.
This effectiveness rate is based on an analysis of results from participants who had received the 2 dose regimen that will be used in Canada. AstraZeneca said its COVID-19 vaccine was 76 effective at preventing symptomatic illness in a new analysis of its major US trial -. In some countries the two-dose AstraZeneca vaccine.
AstraZeneca said its COVID-19 vaccine was 76 effective. 4 hours agoResults from a new US trial of AstraZenecas Covid-19 vaccine may have used outdated information US federal health officials have said in a jolt to the company that has faced scrutiny. On 22 March 2021 AstraZeneca released interim results from the phase III trial conducted in the US that showed efficacy of 79 at preventing symptomatic COVID-19 and 100 efficacy at preventing severe disease and hospitalisation.
Late last year I asked. A clinical trial is being conducted of COVID-19 Vaccine AstraZeneca given to people with stable HIV infection with results expected in a few months. These results suggest that AstraZenecas two-dose vaccine is a little more effective at preventing COVID-19 than the single-shot vaccine made by Johnson Johnson which was 66 effective in preventing disease in its major trial.
1 day agoVials labelled AstraZeneca Pfizer-Biontech Johnson Johnson Sputnik V are seen in this illustration. Following its approval by the European Medicines Agency in January the COVID-19 AstraZeneca vaccine was administered to all adults in Germany. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use.

Astrazeneca Resumes Its Covid 19 Vaccine Trials In The U K Coronavirus Updates Npr

Covid 19 Vaccine Prices Revealed Pfizer Moderna And Astrazeneca Observer

What You Need To Know About Astrazeneca S Covid 19 Vaccine Science In Depth Reporting On Science And Technology Dw 18 03 2021

Covid 19 Good And Not So Good Results For Astrazeneca

Astrazeneca Announces Highly Effective Covid 19 Vaccine Voice Of America English

Astrazeneca Covid Vaccine Shows Positive Results In Lancet Study News Dw 08 12 2020

Pfizer Astrazeneca Vaccines Provide Similar Protection Against Symptomatic Covid 19 Study Science News
Astrazeneca S U S Trial Shows Coronavirus Vaccine Is 79 Percent Effective The Washington Post

Pakistan Approves Astrazeneca Covid 19 Vaccine For Emergency Use Reuters

Astrazeneca Chadox1 S Ncov 19 Recombinant Covid 19 Vaccine
Astrazeneca S Covid 19 Vaccine Holds Up In Updated Data Analysis Science News

Covid Vaccine Astrazeneca S Could Be Distributed In Eu By Mid February

Covid 19 Uk Setting Up Vaccine Centres Ready For Rollout Matt Hancock Bbc News

Tracking The Progress Of Covid 19 Vaccines Center For Data Innovation

Sweden Suspends Use Of Astrazeneca Covid 19 Vaccine Coronavirus Updates Npr

Covid 19 Update The Chadox1 Astrazeneca Covid 19 Vaccine Rebel Em Emergency Medicine Blog

Covid 19 Vaccines Vaccine Knowledge
Austria Suspends Astrazeneca Covid 19 Vaccine Batch After Death

Pfizer Astrazeneca Covid 19 Vaccine Combination Yields Better Results Study Finds Deccan Herald

Post a Comment for "Astrazeneca Covid 19 Vaccine Results"